Low-cost conductivity meter
Water quality is vital for health, agriculture, and ecosystem stability, with electrical conductivity (EC) serving as a key indicator of dissolved ion concentrations and potential contamination. Traditional monitoring tools are often costly, limiting their use in large-scale or resource-constrained settings. This study presents the development of a low-cost, three-electrode EC sensor using the Arduino platform, stainless steel electrodes, and a simple voltage divider circuit. Designed for affordability and replicability, the sensor addresses challenges such as electrode polarization and electrochemical interference, offering a practical alternative for environmental monitoring, especially in underserved regions.
The low-cost conductivity sensor was developed using an Arduino Nano microcontroller with a 10-bit analog-to-digital converter, selected for its compact size, affordability, and compatibility. The system integrates a three-electrode stainless steel probe, a temperature sensor (DS18B20) for temperature compensation, a datalogger with a real-time clock and microSD storage, and a 12V LiPo battery pack for field autonomy. A relay was included to disconnect the electrodes between measurements, reducing electrochemical degradation. All components were assembled inside a waterproof PVC housing, making the sensor portable, low-cost (under $10), and suitable for classroom or field applications.


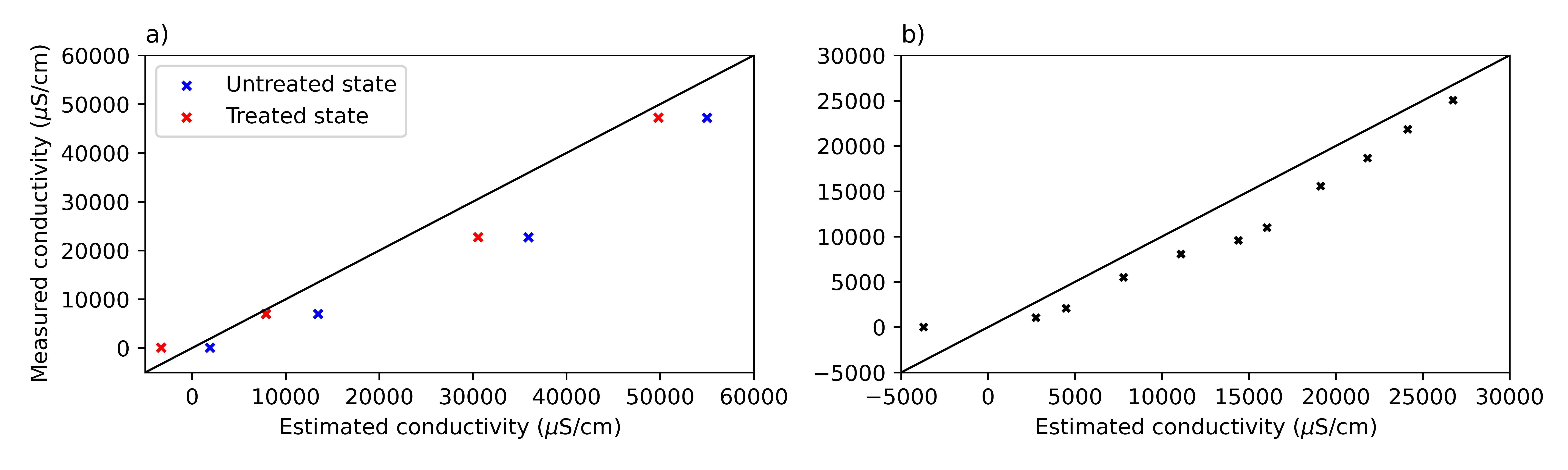
Sensor calibration involved two laboratory setups using untreated and thermally treated electrodes coated with silicone resin to assess the effect of surface treatment on signal stability. Test solutions were prepared by dissolving NaCl in deionized water to obtain a range of known conductivities (100 to 47,000 µS/cm). Readings were recorded and compared with a commercial reference sensor to build calibration curves using different regression models. Validation showed that treated electrodes improved measurement consistency, and performance was further evaluated in a larger tank with gradual conductivity increments. Accuracy was assessed using RMSE and relative error metrics.
The developed low-cost conductivity sensor was tested across a wide conductivity range (14 to 50,000 µS/cm), with output voltage decreasing consistently as conductivity increased, in accordance with the voltage divider principle. The sensor exhibited rapid signal stabilization, especially at higher conductivities, often stabilizing within 5 seconds above 7,000 µS/cm. For lower conductivities, stabilization occurred within 60 seconds. Calibration involved both untreated and treated stainless-steel electrodes, with treated electrodes showing improved accuracy. A segmented calibration model—linear at low conductivities and power-law at higher values—was used to account for the nonlinear voltage-conductivity relationship, yielding excellent fits, particularly with treated electrodes (R² = 0.999).
Validation confirmed the enhanced performance of treated electrodes, with root mean square error (RMSE) reduced from 836 to 448 µS/cm and mean relative error from 56% to 18%. A second test using finer conductivity increments (~250 µS/cm) maintained similar RMSE but showed increased relative error (39%) due to higher sensitivity in low-voltage fluctuation conditions. Despite this, the sensor delivered adequate accuracy for semi-quantitative applications, demonstrating its usefulness for environmental monitoring tasks where rapid detection of conductivity changes is more important than precise absolute values.
Compared to similar low-cost sensors in the literature, which target more limited ranges, the developed device stands out for its broad operational scope and quick response time. While the treated electrodes significantly improved performance, the overall accuracy still lags behind some advanced designs reporting errors under 6%. Nonetheless, the sensor offers a practical solution for field applications, particularly in freshwater systems, where detecting sudden conductivity changes can signal pollution or contamination. Future improvements may include optimized electrode treatments and adaptive calibration methods to further enhance reliability and applicability.